Properties of Matter
There are different kinds of materials and they have different physical properties of matter. These properties help us to recognise them. We touch a material, see its color, smell it and thereby recognise it. The features which help us to recognise a particular material are called properties of the material.
1. Hardness and softness: Some materials are hard and some are soft. Stone is hard while clay and plastic are soft. Some materials keep their shape unchanged when pressed and some do not. Most solids have their shape unchanged when pressed while liquids and gases do not.
2. Transparency: There are some materials which allow the light to pass through. Such materials are called transparent. The materials which do not allow the light to pass through them are called opaque bodies.
Glass-plate, polythene sheet, water, air etc. are the examples of transparent while wooden plank, iron sheet, book, etc. are the examples of opaque.
3. Conductivity of heat: If we take two rods, one of iron and
another of wood and place one end of each in fire, on touching their other
ends, the iron rods feels hot while the wooden one does not. The first end of
the wooden rod actually catches fire. That means the iron rod passes heat from
one end to another while the wooden rod does not.
The ability of a substance to allow the flow of heat through its body is called conductivity of heat or thermal conductivity. The substances which allow the heat to pass through them easily are called good conductors of heat. The substance which do not allow the heat to pass through them easily are called bad conductors of heat. Due to bad conductivity of heat, we use a wooden or plastic handle in a sauce-pan. This handle saves our hand from being burnt due to heat possessed by pan. Wood, plastic, etc. are bad conductors of heat while metals like copper, silver, gold, iron, aluminium, etc. are good conductors.
4. Electrical conductivity: The materials which allow electricity to flow easily through them are called good conductors of electricity. The materials which do not permit electricity to flow through them easily are called bad conductors of electricity. Metals like copper, silver, aluminium, etc. are good conductors of electricity while rubber, wood, porcelain, etc. are bad conductors.
5. Breakability, malleability and ductility: Some of the solid materials like marble, salt, alum, stone, glass, etc. break easily when hit with a hammer. Such materials are breakable or brittle and this property is called breakability or brittleness.
Some materials like copper, brass, soft-iron, etc. do not break but become flat or expand on hammering. These are malleable. This property is known as malleability.
Thus materials like copper, aluminium, silver, etc. expand when hammered. Such materials can be drawn into wares also. These materials are called ductile and the property ductility. The same metals may be malleable and ductile both. We see that electric wires are usually made of copper or aluminium as they have the properties of malleability and ductility both.
6. Color: Some materials are recognised by their colors. Water is colorless, lime is white, turmeric is yellow, leaves are green, copper sulphate blue vitriol is blue, red chilli is red.
7. Smell: Some materials are recognised by their characteristic smell. Kerosene oil, petrol, rose flower, jasmine flower, etc. have their characteristic smells. We say that some substances have a pleasant smell while others unpleasant.
8. Taste: Many materials are recognised on the basis of tastes. Thus we know that lime is sour, sugar is sweet, salt is salty, water is tasteless and neem-leaves are bitter. Certain substances are poisonous and some chemicals are dangerous for health. So we should be careful in tasting such a substance. Everything should not be tasted unknowingly or without permission.
9. Inflammability: Some materials quickly catch fire when heated and start burning. They are called inflammable materials. When we light a gas furnace or a gas-stove, the gas starts burning. When we put a lighted match-stick into kerosene oil, it begins burning. When lighted match-stick is put into glass of water, the stick extinguishes. It is so because kerosene oil is inflammable while water is non-inflammable.
10. Melting and There are some solids which melt on heating wax, sealing wax, ice, frozen ghee, etc. melt into liquids when heated.
There are some solids which on being heated take the form of gas without melting. The process through which a solid vaporises into gas on heating is called sublimation. Camphor, iodine, moth-balls, etc. sublimate on heating.
11. Solubility: If we put some sugar in water, it disappears. When we put sand in water, it does not disappear. We say, sugar dissolves in water and sand does not. Thus, sugar is soluble and sand is insoluble in water. If a substance dissolves in a liquid, it is said to be soluble in that liquid and the liquid is called its solvent. The liquid containing the dissolved substance is called solution of the soluble and the solvent. The soluble substance is also given the name solute.
The substance which does not dissolve in a liquid is said to be insoluble in that liquid.
We know that water is the solvent of a large number of substances. Sugar, salt, alum, blue vitriol, potassium-permanganate, etc. are soluble in water, while wax, sand, paints, etc. are insoluble in it. There are substances which do not dissolve in water, but dissolve in other liquids. Candle-wax, grease, paint, etc. do not dissolve in water but dissolve in kerosene oil or petrol.
A liquid dissolves not only solids but other liquids and gases also. Water dissolves air and carbon-di-oxide. Fish and other water animals take in this dissolved air. When a soda or cold drink is opened, we see carbon-di-oxide fizzling out. Liquid paint dissolves in kerosene oil and alcohol in water.
From Properties of Matter to HOME PAGE
Recent Articles
-
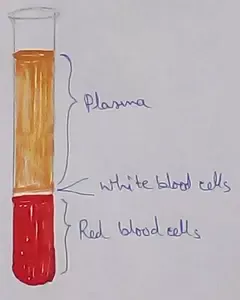
What Is Plasma? | Blood Plasma | Proteins | Nutrients | Cholesterol
Nov 07, 25 10:29 AM
Blood is a mobile fluid which is a connective tissue and is derived from the mesoderm like cell any other connective tissue. Colour of blood is reddish and that flows inside the blood vessels by means… -
Disorders of Respiratory System | Tuberculosis | Pleurisy | Emphysema
Oct 28, 25 11:39 PM
Tuberculosis is very common disease and is caused by a type of bacteria called Mycobacterium tuberculosis. This disease causes different trouble in the respiration and infection of several parts of th… -
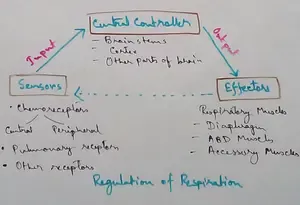
Regulation of Respiration | Respiratory Centres | Inspiratory Area |
Oct 14, 25 12:13 AM
Respiratory Centre is the area that controls the rate of respiration and it is observed to be located in medulla oblongata and pons. Respiratory Centre has the following will dispersed components like… -
Explain Transport of Gases | External Respiration | Tissue Respiration
Oct 09, 25 11:35 PM
In humans gaseous exchange is completed in the following ways the steps are - External Respiration or Breathing - Breathing in false taking in of Oxygen and giving out of carbon dioxide in the body. M… -
Kind and Number of Teeth | Location of Teeth in Mouth | Care of Teeth
Sep 11, 25 12:52 AM
Kind and Number of Teeth



New! Comments
Have your say about what you just read! Leave me a comment in the box below.