Change of State of Matter
We will discuss about the different change of state of matter. We know solid, liquid and gas are the three states of matter.
Does heating and cooling causes changes of state of water?
When we take out a tray of ice from the refrigerator and leave it out for sometimes we see the ice will melt and form water. Now if we boil the water it will evaporate and escape into the atmosphere as water vapour.
Ice is a solid. On heating it becomes water which is a liquid.
A liquid becomes a gas on further heating. On cooling, the opposite changes occur. When water vapour is cooled, it changes to water. When water is cooled, it changes to ice.
Does all solids melt to form liquid and does liquids again change back to form solids?
Let us take three pans. In one pan put a piece of butter. In the other pan put a piece of wax. In the third pan take a piece of iron. Now heat the three pans. After heating we see butter, wax and iron melts but iron has to be heated much more than butter or wax.
Examples to see that some solids dissolve in a liquid to form a solution:
Take water in a glass and add a teaspoon of sugar in it. Stir the water with a spoon. We will see that the sugar slowly disappears in the water. Then we see that the sugar has dissolved in the water. The water with dissolved sugar becomes sweet to taste. This sweet water is called a solution of sugar in water. The sugar is called the solute. The water that dissolves the solute is called the solvent.
Similarly, salt dissolves in water to form a solution that taste salty. Suppose if we dissolve a teaspoon of sand in water we see it does not dissolve because all solids does not dissolve in water.
Volume of liquid before and after dissolving solids:
Let us take water in a glass and mark its level then dissolve two teaspoonful of sugar in it. Now when we check the level of water we find that the level remains the same.
We have already learned that in a liquid the molecules are not so closely packed together. There are spaces between the molecules and when sugar is dissolved in water, the sugar molecules fill these empty spaces. So there is no increase in volume of water.
Suppose if we go on dissolving more and more sugar in the water we find after sometime the sugar stops dissolving and the remaining sugar settles at the bottom of the glass without dissolving. The sugar stops dissolving because all the empty space between the water molecules is now filled with sugar molecules and no more sugar can dissolve. Such a solution is called saturated solution.
From Change of State of Matter to HOME PAGE
Recent Articles
-
Plants Around Us | Big & Small Plants | Shrubs & Herbs | Water Plants
Feb 03, 26 02:01 AM
We see different types of plants around us. Plants are living things. They breathe and grow. They also reproduce. Most of the plants grow on land. Some plants grow in water. -
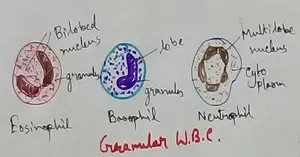
Formed Elements of Blood | Erythrocytes | ESR |Leukocytes |Neutrophils
Jan 15, 26 01:25 AM
Formed elements formed elements are constitute about 45 % of blood afeias haematocrit value packed cell volume mostly of red blood corpuscles and are of 3 types- erythrocytes, leukocytes and blood pla… -
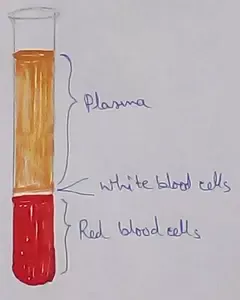
What Is Plasma? | Blood Plasma | Proteins | Nutrients | Cholesterol
Nov 07, 25 10:29 AM
Blood is a mobile fluid which is a connective tissue and is derived from the mesoderm like cell any other connective tissue. Colour of blood is reddish and that flows inside the blood vessels by means… -
Disorders of Respiratory System | Tuberculosis | Pleurisy | Emphysema
Oct 28, 25 11:39 PM
Tuberculosis is very common disease and is caused by a type of bacteria called Mycobacterium tuberculosis. This disease causes different trouble in the respiration and infection of several parts of th… -
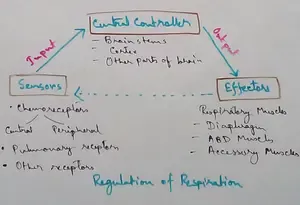
Regulation of Respiration | Respiratory Centres | Inspiratory Area |
Oct 14, 25 12:13 AM
Respiratory Centre is the area that controls the rate of respiration and it is observed to be located in medulla oblongata and pons. Respiratory Centre has the following will dispersed components like…




New! Comments
Have your say about what you just read! Leave me a comment in the box below.