Dry Electrical Cell
A dry electrical cell is a small cylindrical or box shaped container full of some chemical mixture. It can generate low power electrical energy. We commonly call it a battery.
A battery is made up of a hollow cylindrical zinc container with one end open. It is filled with a mixture of ammonium chloride paste, dust of manganese dioxide and graphite. A carbon rod is inserted through the centre of the mixture. The bottom of the carbon rod is kept a little above the base of the zinc container. A brass cap is fitted at the top of the carbon rod. The upper part Cross Section of a Battery of the container is first sealed with molten bitumen and then with a plastic lid. The plastic lid has a hole at its centre, through which the brass cap emerges out. After that the outer zinc container is wrapped with thick paper and then with a metal sheet. The cell is now ready for use. Batteries are commonly used in torches, radios, calculators, time pieces, tape recorders, electrical toys etc.
Now we
will discuss about the proper method of using dry electrical cells or batteries.
A dry electrical cell has two terminals. The top brass cap is its positive
terminal and the exposed portion of the outer zinc container is its negative
terminal. If you properly connect the two terminals of a battery within a
torch, the battery will supply electrical energy and the torch will glow. You
can also multiply the supply of energy or current electricity by using two or
more batteries. For this purpose you have to maintain the proper direction to
arrange the batteries in a series.
When you insert a battery in a torch, put the brass cap of the first battery towards the bulb end. Then insert the next one in the same way so that the back of the first battery touches the top brass cap of the second battery. If more than two batteries are used, you have to apply the same method. In modern appliances, the directions of terminals are marked as (+) and (-) signs. In that case put the top brass cap towards the (+) mark.
We should not play with current electricity. Electricity is a very strong form of energy. So never handle it carelessly. Current electricity flows through all metals and non-metals like graphite. It also flows through our body. Well may you be surprised that a torch battery generates electricity but we can handle it so easily. It is because a torch battery has a very little electrical power in it, so it does not cause any harm to us. But the domestic electrical lines carry very powerful electrical energy. Such a strong energy may lead to death if that flows through our body for a few seconds only. So never touch any open and live electrical wire or insert any metal body in a live plug point. Always keep electrical points dry, never touch switches or plug points with wet hands. Do not try to remove a kite from overhead electrical lines. Repair damaged electrical appliances immediately. Do not tap power from the electrical lines of your house for any experiment. Never try to rescue any electrified person with bare hand; always stand on a wooden board or wear a rubber sandle before doing so.
From Dry Electrical Cell to HOME PAGE
Recent Articles
-
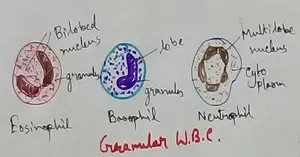
Formed Elements of Blood | Erythrocytes | ESR |Leukocytes |Neutrophils
Jan 15, 26 01:25 AM
Formed elements formed elements are constitute about 45 % of blood afeias haematocrit value packed cell volume mostly of red blood corpuscles and are of 3 types- erythrocytes, leukocytes and blood pla… -
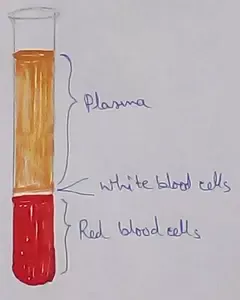
What Is Plasma? | Blood Plasma | Proteins | Nutrients | Cholesterol
Nov 07, 25 10:29 AM
Blood is a mobile fluid which is a connective tissue and is derived from the mesoderm like cell any other connective tissue. Colour of blood is reddish and that flows inside the blood vessels by means… -
Disorders of Respiratory System | Tuberculosis | Pleurisy | Emphysema
Oct 28, 25 11:39 PM
Tuberculosis is very common disease and is caused by a type of bacteria called Mycobacterium tuberculosis. This disease causes different trouble in the respiration and infection of several parts of th… -
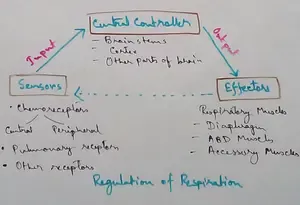
Regulation of Respiration | Respiratory Centres | Inspiratory Area |
Oct 14, 25 12:13 AM
Respiratory Centre is the area that controls the rate of respiration and it is observed to be located in medulla oblongata and pons. Respiratory Centre has the following will dispersed components like… -
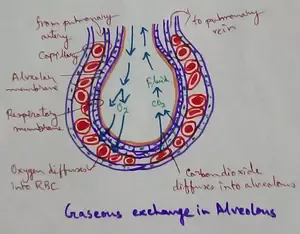
Explain Transport of Gases | External Respiration | Tissue Respiration
Oct 09, 25 11:35 PM
In humans gaseous exchange is completed in the following ways the steps are - External Respiration or Breathing - Breathing in false taking in of Oxygen and giving out of carbon dioxide in the body. M…

New! Comments
Have your say about what you just read! Leave me a comment in the box below.