Plant-Water Relations
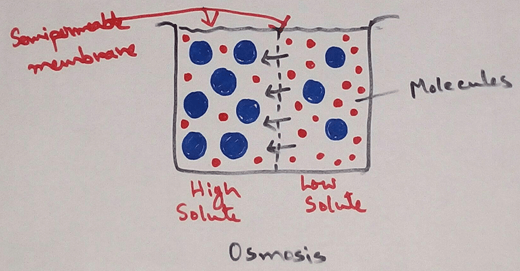
Definition of Osmosis - This is a physical phenomenon of net flow of solvent molecules from a solution of lower concentration to a solution of higher concentration through a semi permeable membrane (a membrane which allows only the solvent molecules and not the solute molecules to pass freely through it is called semi permeable membrane) which separates the two solution or we can say - Osmosis is the physical process by which net diffusion of solvent molecules across a semi-permeable take place.
Osmosis in Biological Systems: In biological system osmosis occurs between the fluid present inside and outside the cells where plasmalemma act as semipermeable membrane. It permits water but not many solutes including proteins to pass freely through the membrane but in true sense the plasmalemma is not a perfectly semipermeable membrane because a few solute mein diffuse through it. For this reason the plasmalemma is designated as a selectively or differentially permeable membrane. Osmosis takes place through plasma membrane whenever the intracellular fluid and extracellular fluid differ in concentration. According to some scientist Osmosis in biological field means diffusion of water across the plasma membrane, in response to a concentration gradient.
Types of Osmosis: Depending upon the direction of flow of water osmosis can be divided into two - endosmosis and exosmosis.
Endosmosis is a process where entry of water into a cell from its surrounding occured in the intracellular fluid become more concentrated than the extracellular fluid.
Exosmosis process takes place when intracellular fluid becomes less concentrated than the extracellular fluid and water flows out of the cell.
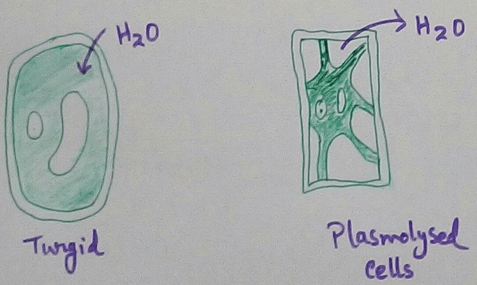
Plant cells are protected from osmotic deformities like shrinkage swelling etc due to the presence of a rigid cell wall.In plant cells prolonged exosmosis may lead to shrinkage of Protoplasm which retreats from the cell wall and accumulate centrally this is known as plasmolysis.
Explanation of the Mechanism of the Process Osmosis:
When the two solutions of different concentrations are separated by a semipermeable membrane there occurs the net flow of solvent from the less concentrated solution to the more concentrated one. In fact the solvent water molecules move in both directions across the semi permeable membrane but since the number of solvent molecules moving from the dilute to the concentrated side is much greater than that in the reverse direction there is a net flow of solvent from the dilute to the concentrated side.Osmosis is a special type of diffusion process by which the solvent molecules pass across a semipermeable membrane from a region having greater number of solvent molecules to the region having smaller number of solvent molecules.
The mechanism of osmosis can be explained on the basis of binding between solute and solvent molecules in a solution. If there is higher number of solute molecules of a solution then the number of solvent molecules bind why the solute will be more. Which results to remain the few number of free solvent particles available for random motion. The least is the concentration of a solution the more will be the random motion of free solvent molecules in it. Osmosis continuous until an equilibrium is reached when the two solutions at a same concentration of the rise of hydrostatic pressure on the concentrated side becomes high enough to prevent further entry of solvent into this compartment.
Physiological Importance of Osmosis in Plants -
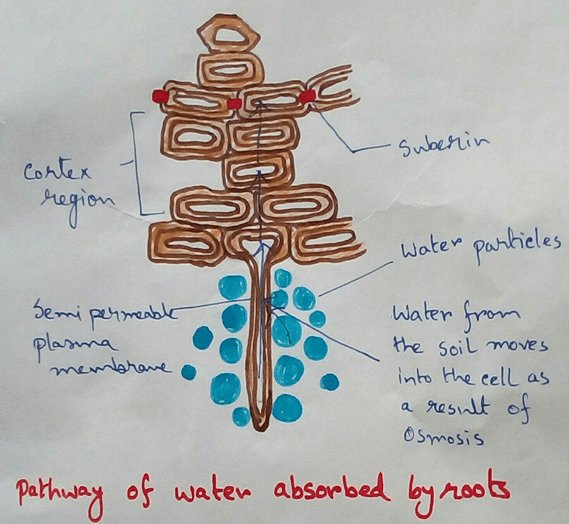
* Absorption of water from soil by the root hair of plants occurs by the process of osmosis.
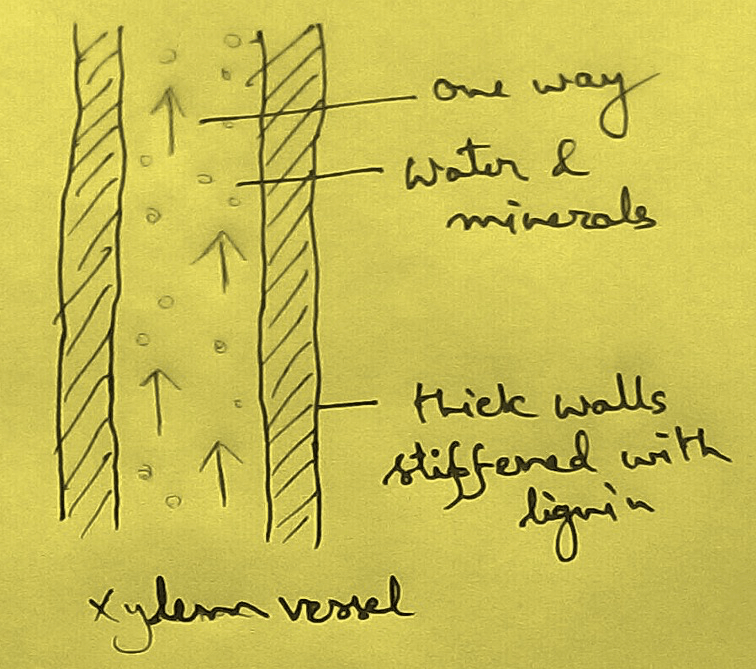
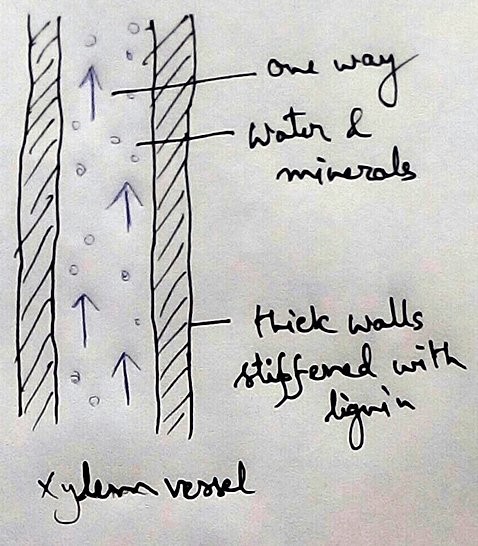
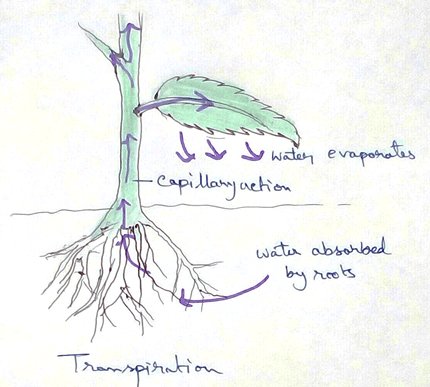
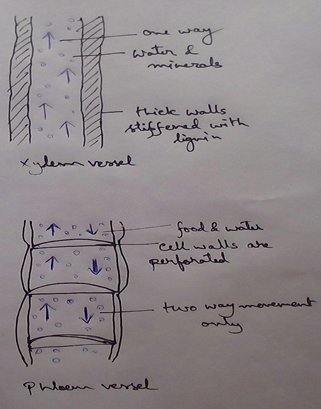
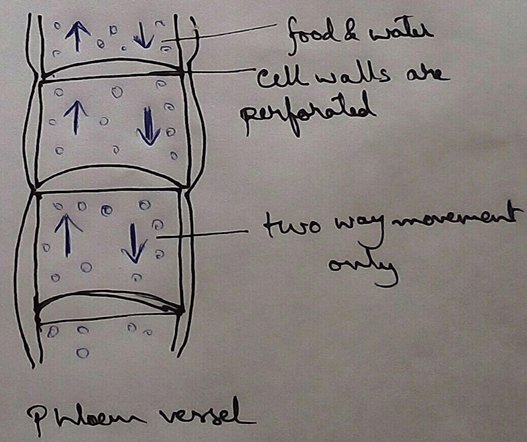
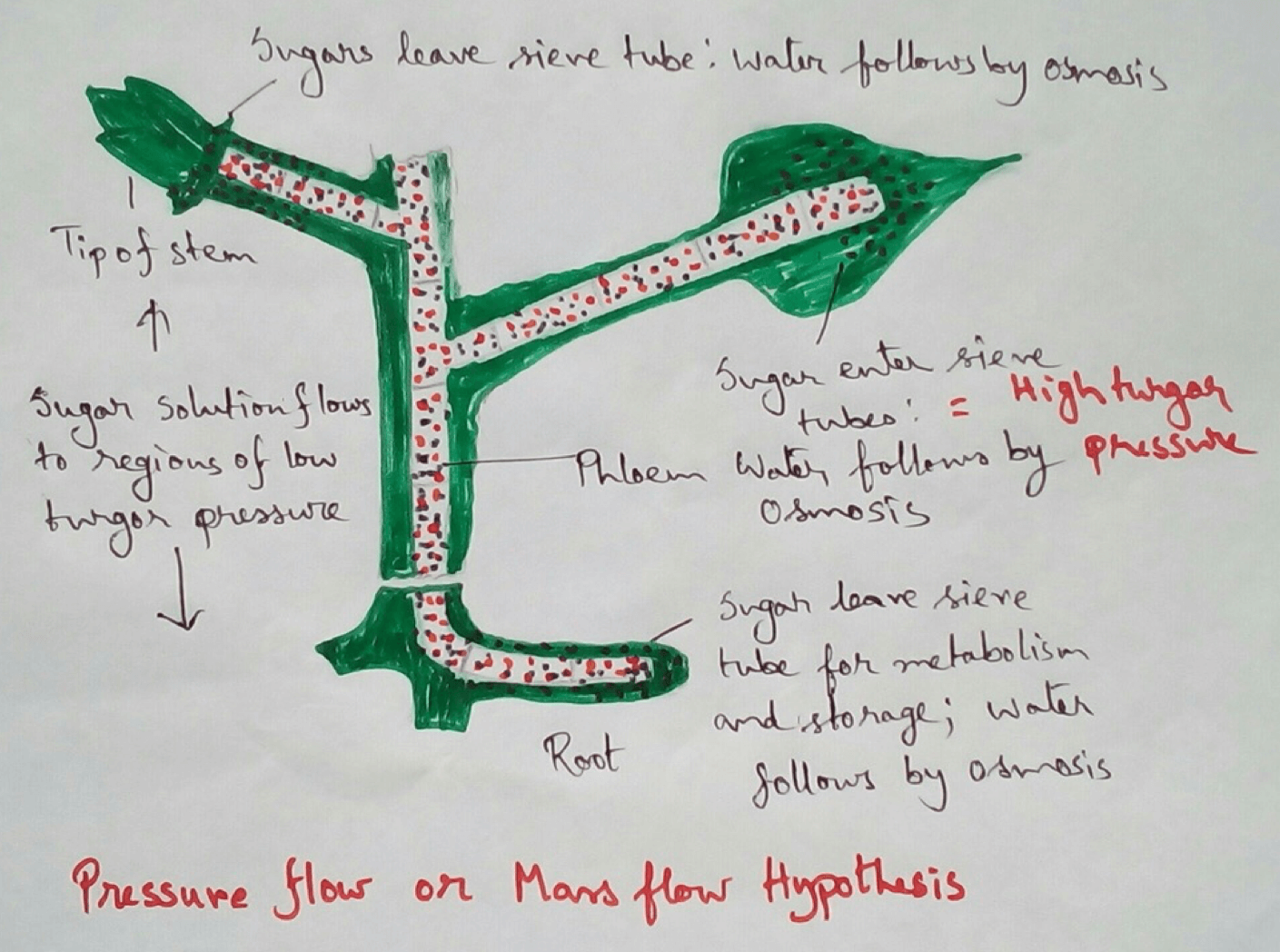
* This absolute water by the root hairs are transported to the cortical cells and then through thexylem is a two different parts of the plant by the cell osmosis.
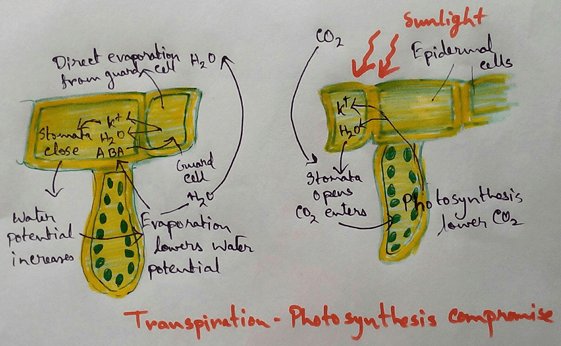
* Endosmosis in plant cells create turgor pressure which helps in several functions like control of stomatal opening and closing,growth of meristematic tissue and various type of seismonastic movement.
* This also profile rigidity and mechanical support to soft plant organs and help theherbs to stand erect due to the turgor pressure in their cells.
Water Potential - Water potential of a solution is defined as the difference between the free energy of water molecules in pure water and the free energy of water in the solution. It is denoted by the Greek letter psi and is measured in bar.The term water potential was first introduced in 1965 Stayler and Taylar to express the free energy of water in a solution.1 bar = .987 atm. or 10 6 Dynes/ centimetres square.
The water potential of pure water is zero and it is lowered by adding solute molecules this occurs because of the free energy of water is decreased by the presence of solute molecules are ions that collide with the water molecules.Thus water potential of a solution is dependent on the concentration of the solution and the value of it is always less than zero hence it is negative.
Factors affecting water potential- increase in concentration of a solution is always becomes more negative and vice versa for the value of water potential as water potential is inversely proportional to the concentration of a solution.
Plasmolysis - Plasmolysis is a phenomenon in which contraction of protoplasm of a living plant cell occurs due to exosmosis. Contraction or shrinkage of the Protoplasm due to loss of water from the cell read make the cell wall more rigid and less elastic which cannot keep pace with the shrinkage of Protoplasmh, encee the cell membrane is pulled away from the cell wall. Then for the loss of water from the cell contents produces more contraction of Protoplasm and Fitch finally recedes completely from the cell wall and assumes a spherical shape. Sales at this condition are called plasmolysed condition.
In the plasmolysed cell the space between the cell wall and the contract is Protoplasm is occupied by the external hypotonic solution + water which has diffuses out of the protoplasm.At the initial stage of plasmolysis when the cell wall has reached its limit of contraction but the Protoplasm has not yet received it from the cell wall is called incipient plasmolysis. Means incipient plasmolysis can be defined as the stage of plasmolysis at which the first sign of shrinkage of Protoplasm is detectable. If a plasmolysis cell is placed in hypotonic solution or distilled water then endosmosis occurs and the cell regain seeds turgidity and original shape and size. This process is called deplasmolysis as deplasmolysis can be defined as reverse of plasmolysis.
Imbibition:
Definition of Imbibition - Imbibition is type of diffusion process when water is absorbed by the solid colloids and it increases in volume. Water potential gradient is important between theabsorbent and the water or solution that will be imbibed.
This process increases the volume of the imbibed material as a result it increases imbibition pressure. Sometimes this pressure can be of very high magnitude so that it is capable of destroying the rocks by placing dry woods inside the services of the rocks. Skin grafting also received oxygen and nutrition in this process.
● Importance of imbibition in biology-
● By this process water is observed by the seeds.
● This process also causes absorption of water by the dry woods.
Without imbibition seedlings were not able to come out from the underground and raise themselves in the environment.
Herb or seedlings do not have a well developed mechanical tissue for their support . They Stand erect by virtue of turgidity of the cells caused by water absorption. Because root hair spontaneously absorb water from the soil and pass it to all the cells by a osmosis as the result of which the cells of the heart or Seedling become target and tightly packed which gives mechanical support to the plant for standing erect.
Comparison between Osmosis and Plasmolysis:
Plasmolysis causes accumulation of protoplasm centrally as it retreat from the cell wall. On the other hand osmosis is not associated with the accumulation of Protoplasm within cell it only allows some specific solute or water to pass through it.
Comparison between Isotonic, Hypertonic, Hypotonic Solution:
Isotonic Solution - A solution is called isotonic when it exerts no osmotic effect on the living cells. Examples –cells of placed in a solution where there is absence of exosmosis or endosmosis.
Hypertonic Solution - This is a type of solution in which if cells are placed exosmosis takes place.
Hypotonic Solution - This is a solution in which if cells are kept then it causes endosmosis or inflow of water into the cell causes the cell to sweal. Further increase of pressure may causes lysis of cell.
Examples - If RBC are placed in the hypotonic saline water, it ruptures and the empty envelope is called RBC ghost.
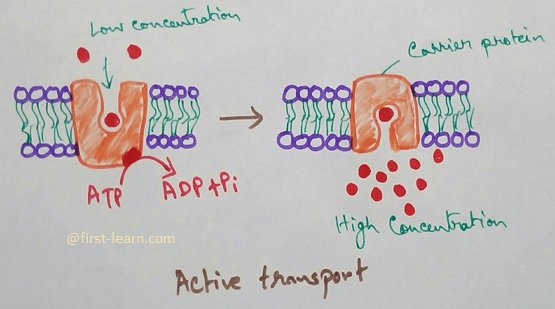
Comparison between Osmosis and Absorption:
Osmosis is a process of passage of water through a semipermeable membrane from a solution of lower concentration to higher concentration but absorption is the process of entry of materials into a cell. In the process of osmosis only water molecules pass through the membrane by a passive process and does not require any carrier. In absorption both water and solids can pass through cell membrane Bajar active or passive process and may not require carrier.
From Plant-Water Relations to HOME PAGE
Recent Articles
-
Plants Around Us | Big & Small Plants | Shrubs & Herbs | Water Plants
Feb 03, 26 02:01 AM
We see different types of plants around us. Plants are living things. They breathe and grow. They also reproduce. Most of the plants grow on land. Some plants grow in water. -
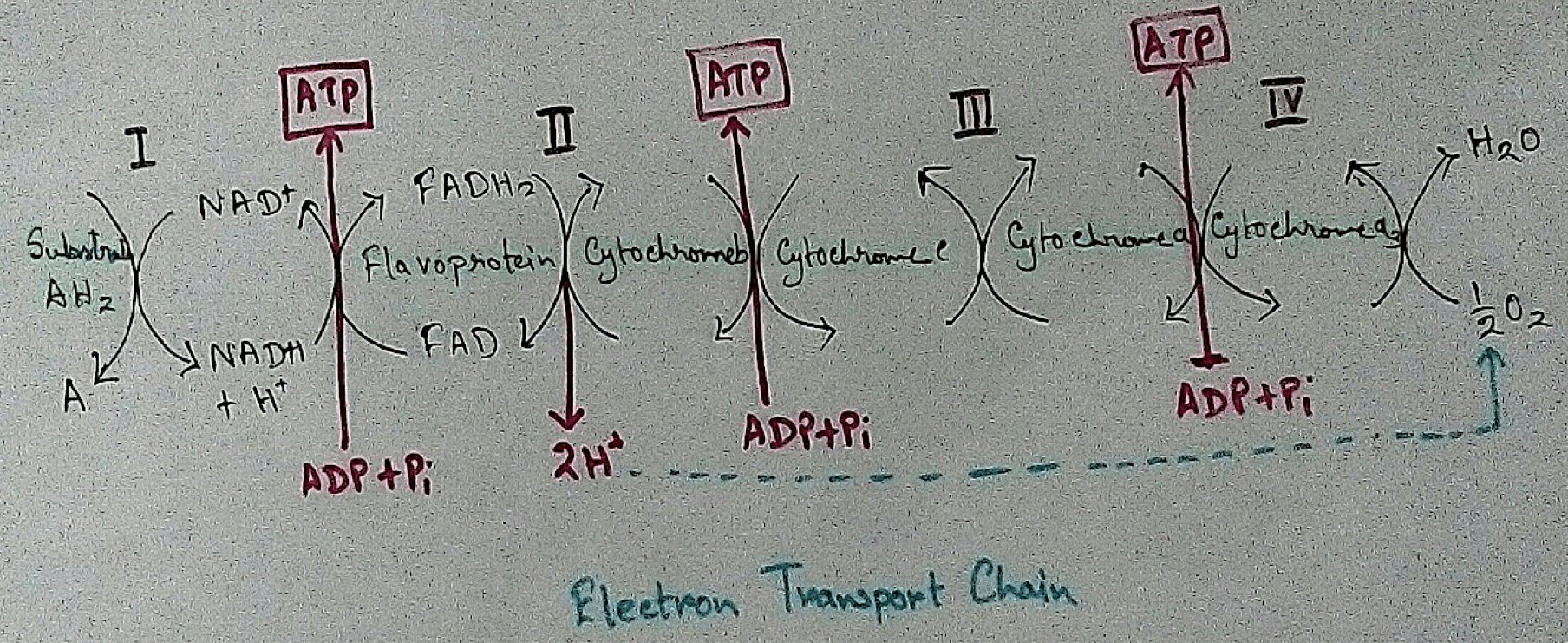
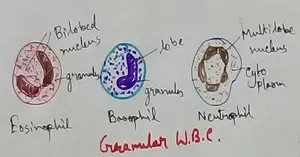
Formed Elements of Blood | Erythrocytes | ESR |Leukocytes |Neutrophils
Jan 15, 26 01:25 AM
Formed elements formed elements are constitute about 45 % of blood afeias haematocrit value packed cell volume mostly of red blood corpuscles and are of 3 types- erythrocytes, leukocytes and blood pla… -
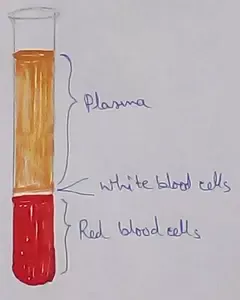
What Is Plasma? | Blood Plasma | Proteins | Nutrients | Cholesterol
Nov 07, 25 10:29 AM
Blood is a mobile fluid which is a connective tissue and is derived from the mesoderm like cell any other connective tissue. Colour of blood is reddish and that flows inside the blood vessels by means… -
Disorders of Respiratory System | Tuberculosis | Pleurisy | Emphysema
Oct 28, 25 11:39 PM
Tuberculosis is very common disease and is caused by a type of bacteria called Mycobacterium tuberculosis. This disease causes different trouble in the respiration and infection of several parts of th… -
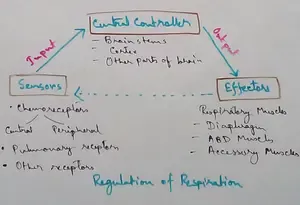
Regulation of Respiration | Respiratory Centres | Inspiratory Area |
Oct 14, 25 12:13 AM
Respiratory Centre is the area that controls the rate of respiration and it is observed to be located in medulla oblongata and pons. Respiratory Centre has the following will dispersed components like…


New! Comments
Have your say about what you just read! Leave me a comment in the box below.