Explain Chemical Reaction
Definition of chemical reaction- Chemical reactions are process where a set of chemical compounds converted to the new set of compound.
In this process, the group of substances that involved in the reaction are called reactants and the new compounds that produces are called products. In chemical reactions only exchange of electrons between the components and breaking of chemical bonds take place. It is either endergonic or endergonic reactions. There is no change of nuclei ,means number proton and neutron remain unchanged.
Example: CO2 + H2O -> C6 H12O6
Here carbon dioxide is reacting with water to produce totally different compound called glucose. So, carbon dioxide and water are reactants and glucose is the product. But the properties of the reactants are different from products.
Types of chemical reaction: Generally chemical reactions can be divided into-
1. Combination – Combination is a type of reaction where two or three components are combined together to form new compound. Here new chemical bonds are formed to prepare new substances.
Example of combination reactions are : H2 +Cl2 → 2HCl
Here hydrogen and chlorine are combined together are called hydrogen chloride.
2. Decomposition - Decomposition is an endorsement thermic reaction where single compound is degraded into two or more components. Here energy is required to break down the chemical bonds. It is of three types – thermal decomposition, electrical decomposition, photogenic decomposition.
Examples of decomposition reactions are-
H2O → H2 + O2 . In presence of electrolysis water is decomposed to form hydrogen and oxygen.
3. Single Displacement - Single displacement reactions are type of reaction where one element of a compound is replaced by the other element.
Examples of displacement reactions are-
Cu + ZnCl2 → CuCl2 + Zn
In this reaction copper is an element which replaces zinc. As a result zinc chloride is converted into copper chloride and zinc is separated.
4. Double Displacement - Double displacement is a type of reaction where cations and anions means positive and negative ions are switch their position to produce new compounds.
Examples of displacement reactions are –
AgNO3 + NaCl → AgCl + NaNO3.
In this reaction two compounds are silver nitrate and sodium chloride are made up of positive ions sodium and silver; negative ions are nitrate and chloride. Here positive ion silver is combined with chloride (negative ion) and produces new compound silver nitrate. On the other hand positive ion sodium combined with negative ion nitrate to form sodium nitrate (a new compound).
5. Combustion - Combustion is a chemical reaction where hydrocarbons are oxidized in presence of oxygen to form carbon dioxide and water.
Examples of combustion reactions are-
CH4 + O2 → CO2 + H2O
In this reaction methane undergoes combustion in presence of oxygen to produce carbon dioxide and Water.
6. Redox - It is a chemical reaction where there is transfer of electrons from one species to other takes place.
Examples of redox reactions are-
Zn + Cu++ → Zn++ + Cu.
Reducing agent + oxidizing agent → oxidised + reduced .
From Explain Chemical Reaction to HOME PAGE
Recent Articles
-
Plants Around Us | Big & Small Plants | Shrubs & Herbs | Water Plants
Feb 03, 26 02:01 AM
We see different types of plants around us. Plants are living things. They breathe and grow. They also reproduce. Most of the plants grow on land. Some plants grow in water. -
Formed Elements of Blood | Erythrocytes | ESR |Leukocytes |Neutrophils
Jan 15, 26 01:25 AM
Formed elements formed elements are constitute about 45 % of blood afeias haematocrit value packed cell volume mostly of red blood corpuscles and are of 3 types- erythrocytes, leukocytes and blood pla… -
What Is Plasma? | Blood Plasma | Proteins | Nutrients | Cholesterol
Nov 07, 25 10:29 AM
Blood is a mobile fluid which is a connective tissue and is derived from the mesoderm like cell any other connective tissue. Colour of blood is reddish and that flows inside the blood vessels by means… -
Disorders of Respiratory System | Tuberculosis | Pleurisy | Emphysema
Oct 28, 25 11:39 PM
Tuberculosis is very common disease and is caused by a type of bacteria called Mycobacterium tuberculosis. This disease causes different trouble in the respiration and infection of several parts of th… -
Regulation of Respiration | Respiratory Centres | Inspiratory Area |
Oct 14, 25 12:13 AM
Respiratory Centre is the area that controls the rate of respiration and it is observed to be located in medulla oblongata and pons. Respiratory Centre has the following will dispersed components like…
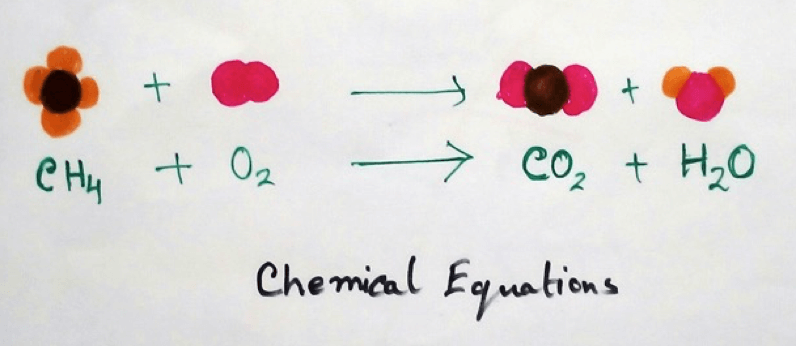




New! Comments
Have your say about what you just read! Leave me a comment in the box below.